Hey, guys today I will be sharing what we have currently been doing in Science. The topic we are learning is chemistry, acids and bases. We learnt about the periodic table, chemical equations, balancing chemical equations, the pH scale, neutralisation and alkalines. Today I will be writing about Acid Rain and the problems it causes for the environment and also about an experiment we did showing the effects of acid rain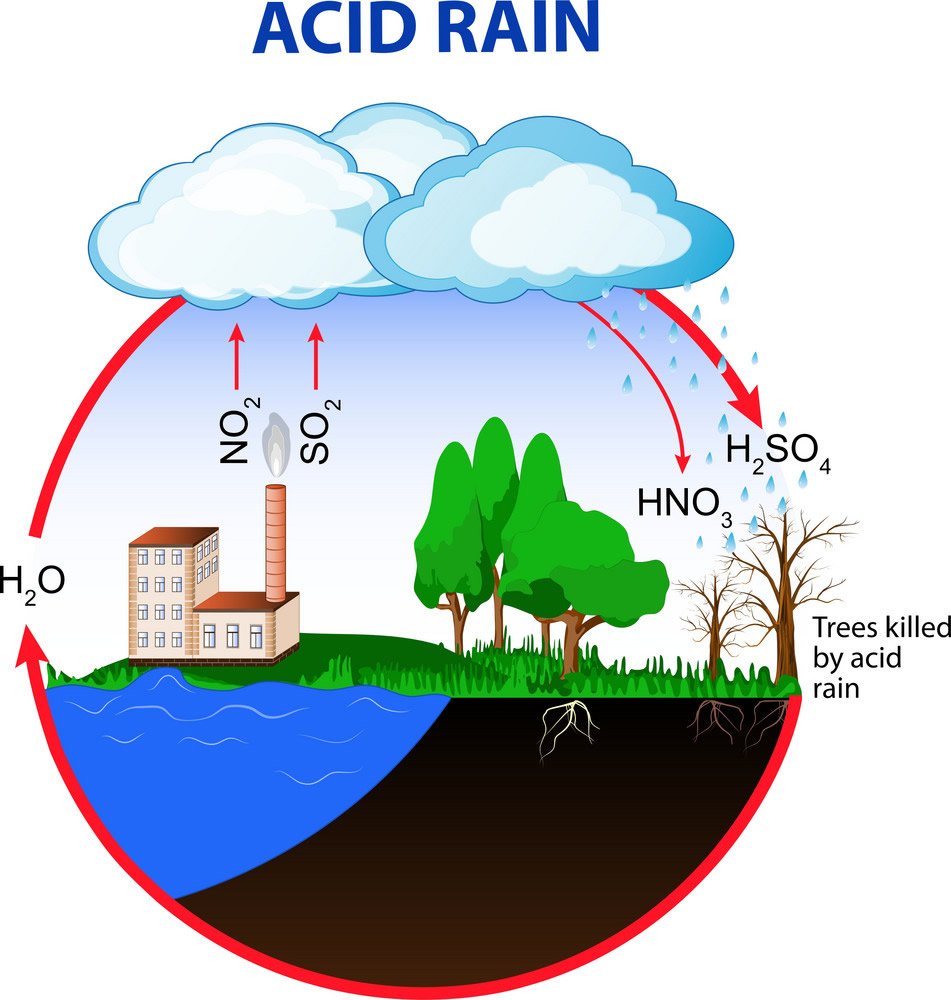
Acid rain is fossil fuels that burn from cars and factories releasing natural gases, coals, and oils into the air. For instance, carbon dioxide (CO2), nitrogen oxide (NO2) and sulfur dioxide (SO2) are some of the harmful chemicals affecting our environment. These molecules dissolve in the air making rainwater in clouds; this would be called a physical change because the state of matter is being changed from a gas to a liquid. After the rainwater is absorbed in the clouds precipitation occurs. Chemicals like nitric acid (HNO3), carbonic acid (H2CO3), and sulfuric acid (H2SO4) are released. The issue here is that the rain is more acidic than usual because of the burning of fossil fuels; this leads to numerous environmental problems like corroding buildings, acidic lakes and the harm of living things.
Acid rain damages many objects like buildings, statues, monuments and cars. The chemicals found in acid rain can cause paint to peel and status to appear worn out; this reduces their beauty and value. Acid rain is extremely harmful to our forestry it causes aluminium (Al) to be released into the soil which makes it difficult for trees to take up water. Nutrients like calcium (Ca) and magnesium (Mg) help make healthy trees but with fewer nutrients, our forestry is getting more infected and weaker. If this continues we will see weak forestry and the environment will change. Acid rain causes many lakes and streams to have a lower pH meaning more acidic. Aluminium (Al) is released into the soil which ends up in our waters, this is very really deadly to our sea creatures. This also touches back on ocean acidification. Rain’s usual pH is 6 and when it is acidic it can go to more than 1000 times more acidic to a pH of 3.
Last week we did an experiment observing the effects of acid rain. This experiment was led by the teacher and was very useful because it helps me understand how rain acid is acidic and what makes it acidic.
Equipment:
- 100 mL Beaker
- Matches – Factories and Cars
- Cotton Wool Balls – Clouds
- Water – Rainwater
- Tape
- Rubber Band
- Lid
- Universal Indicator Solution
Hazards and Safety:
- Protective Eye Wear
- Corrosive (damage or destroy other substances)
Method:
- Firstly, soak the cotton wool balls with water and place them at the bottom of the 100mL beaker.
- Secondly, wrap the matches with a rubber band and tape them to the edge of the beaker.
- To begin the experiment light the group of matches attached to the rim of the beaker.
- Next, quickly cover the beaker with a lid and wait for a few minutes.
- After letting the cotton wool balls absorb the gases lift the lid and let the excuse gas escape.
- Then drain the cotton wool balls into the same beakers, leaving only a light brown solution in the beaker.
- Finally, to get if the solution is a base or an acid pour a few drops of universal indicator.
- WATCH THIS VIDEO AND SEE HOW THE EXPERIMENT WAS SET UP AND DONE!
Observation:
When observing the experiment I noticed that when we put the lid on the beaker it made very polluted gases and was a brownie grey colour. After draining out the soaked up cotton wool balls into the beaker the liquid was very light brown and when adding the universal indicator the solution turned red meaning it was acid. The particular reason for the circumstance is that the chemicals that were made when lighting the matches we absorbed in the cotton balls made a very harmful and acidic solution. The pH value of the solution at the end of the experiment was 3.
New Zealand Oamaru Stone
Acid rain is already is bad but in New Zealand, Oamaru Stone is a very pure limestone and one of the most important building stones. The problem is that it’s easily sawn and work which makes it very susceptible (allowing) to effects pf acid rain. Some effects can be damaging for the stone and can make them appear worn down. Chemicals in acid rain that are damaging are nitrogen oxide (NO2) and sulfur dioxide (SO2).
A solution to Acid Rain:
A solution to this can be reducing our use of fossil fuels, coal, oils, and natural gases. A great way to reduce acid rain is to produce energy without using fossil fuels. Instead, people can use renewable energy sources, such as solar and wind power. Renewable energy sources help reduce acid rain because they produce much less pollution.
5 Effective Ways You Can Prevent Acid Rain
- Purchase or install renewable energy for home use.
- Switch to the battery- or electric-operated lawn mowers and edgers.
- Drive an electric vehicle.
- Switch to an electric fireplace or electric stove (not gas or wood-burning)
- Switch to an electric grill (not gas or wood)
I hope you enjoyed my blog post about acid rain. I really enjoyed learning about this subject because it helped me understand the underlining problems of using fossil fuels, not just climate change. What is something you are doing to help with climate change or acid rain? Bye!
Amazing work Risha, this blog is a great example of your hard, focused work. I am impressed by the many sections it contains, from research on the different gases in pollution that cause acid rain, to Omararu stone, and then the experiment demonstration in class. Your 5 ways to reduce acid rain and environmental affects are very good ideas! Maybe I should look into getting a hybrid car one day to reduce emissions. Electric powered rather than gas powered tools is a great idea. My question to you is, what can NZ do to supply more electricity to the country that doesn’t come from fossil fuels. Excellent work again Risha, 10/10.